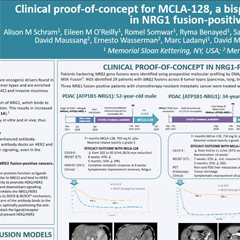
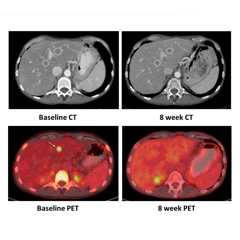
NRG1 Fusions
Different types of cancer can sometimes be distinguished by changes to certain genes. Tumors with certain abnormal changes to a gene may respond differently to cancer treatments.
Certain kinds of alterations in the NRG1 gene are called NRG1 Fusions and may cause increased levels of the growth factor NRG1, which is capable of leading to activation of tumor growth. NRG1 Fusions have been identified in patients with different types of solid tumors, including non-small cell lung cancer (NSCLC), pancreatic cancer, gallbladder cancer, renal cell carcinoma, bladder cancer, ovarian cancer, breast cancer, neuroendocrine tumor, sarcoma, and colorectal cancer.
Source: Cell Volume 33, Issue 5, May 2018
About MCLA-128
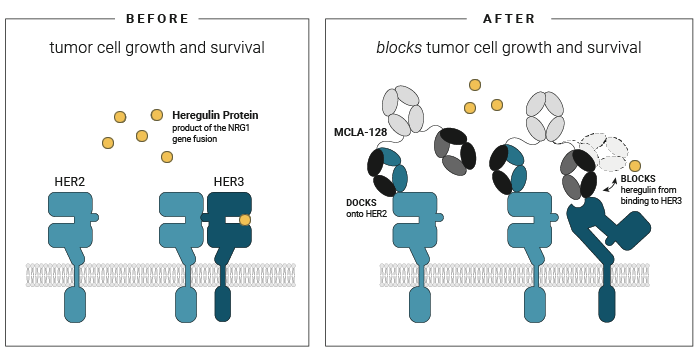
Zenocutuzumab
Geuijen et al. (2018) Cancer Cell
Zenocutuzumab
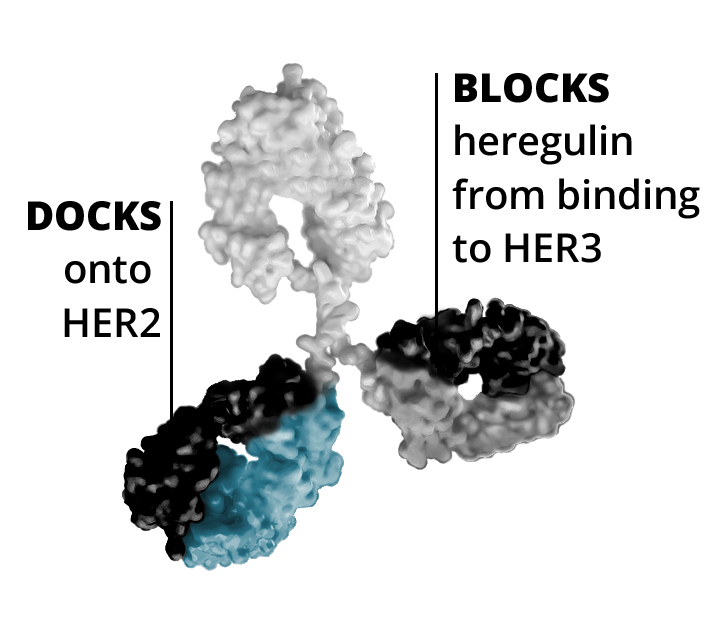
- First, zenocutuzumab
(MCLA-128) docks onto HER2. - Then, it is able to block HER3’s ability to bind NRG1.
When zenocutuzumab
In addition to its direct action on the cancer cell linking to HER2 and HER3, zenocutuzumab
External links related to NRG1 Fusions and MCLA-128
For more information about cancer generally and the availability of potential treatments, please visit the following:
National Institutes of Health – National Cancer Institute